Investigación
Molecular hybrid nanoparticles for biomedical and bioimaging applications
The “bottom-up” modular construction of 1D, 2D, and 3D nanomaterials with molecular precision at the nanometer scale is of paramount interest for the fine-tuned control of their macroscopic properties. For this endeavor, it is essential to have a ready and scalable access to a set of structurally well-defined, homogenous nano-building-blocks with diverse functionality as well as a set of efficient chemical reactions (ideally “click”-type) for their functionalization. Within the last two decades, polyhedral oligosilsesquioxanes (POSS) have emerged as an increasingly important group of 3D nano-building-blocks for the preparation of a variety of hybrid functional-materials. POSS are nanometer-sized cage-like molecules with a core-shell composition consisting of a siloxane inorganic scaffold decorated with organic substituents at the vertices. Due to their rigid inorganic core, these unique nanometer-sized hybrid molecules have superior mechanical and thermal stabilities that are partially transmitted to their derived materials. Applications in areas as diverse as polymers, composite materials, dendrimers, optical materials, coatings, liquid crystals, metal catalysts, drug carriers, and tissue engineering have been described, particularly in the patent literature. POSS are usually synthesized by hydrolytic condensation of organosilicon monomers RSiX3 (R = organic group; X = halogen or alkoxide group) and can be readily modified both on the inorganic cage and the peripheral organic functionality (mono-, multi-, homo- or heterofunctionalized).

The most promising POSS monomers are the highly symmetrical and topologically ideal cube-octameric frameworks (T8), of general formula type (RSiO1.5)8. Key to all potential uses of POSS basic monomers is the ease with which their pendant organic functionality can be altered in a controlled and highly efficient way to produce new molecules suitable for further functionalization. Since these T8 molecules contain eight points for functionalization and partial transformation produces complex mixtures of products that are generally difficult to separate, it is critical that the reaction chosen for this task proceeds in a very efficient way. A relatively wide range of derivatization reactions are suited for this purpose, although care must be taken to avoid strongly acidic, basic, or oxidizing environments that can compromise the stability of the POSS cage. Among these reactions, olefin cross-metathesis, copper-catalyzed azide-alkyne cycloaddition and thiol-ene photoaddition have proven to be the most useful.
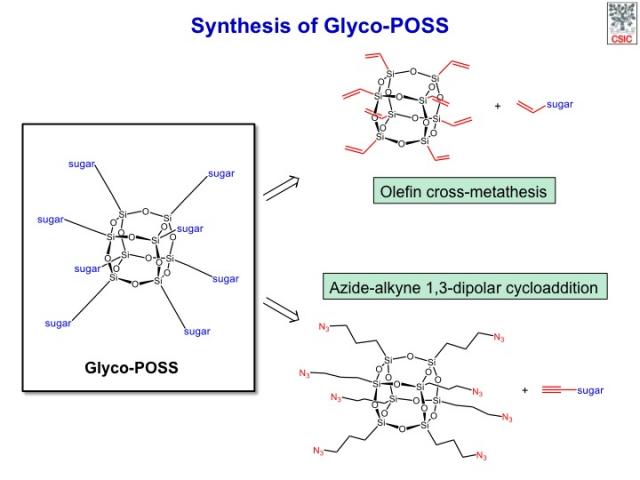
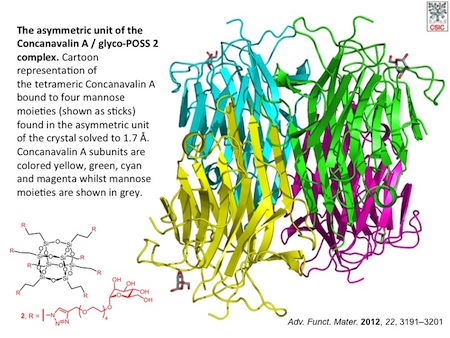
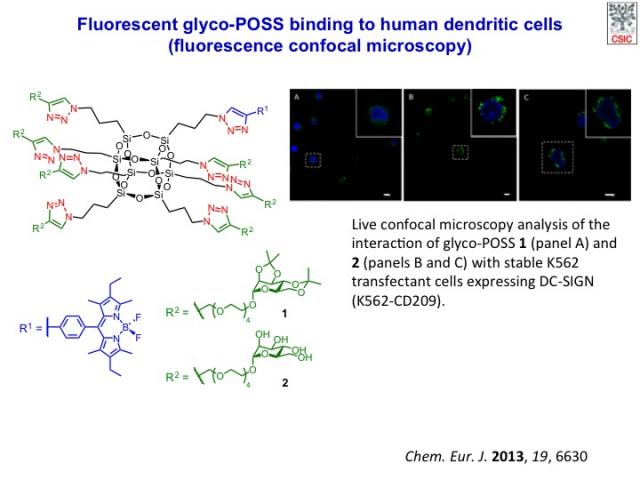
POSS have recently attracted attention as scaffolds for the synthesis of multivalent bioconjugates. We have developed the synthesis of a series of glycosyl-octasilsesquioxanes (glyco-POSS) using a copper(I)-catalyzed azide-alkyne 1,3-dipolar cycloaddition approach is reported. The problems associated with the use of bases or aqueous media in their preparation were investigated and a comprehensive study of the multivalent interaction between the mannosyl-octasilsesquioxanes and a model lectin, concanavalin A (Con A), using an array of complementary biophysical techniques has been carried out. The possibility to modulate the half-life of POSS conjugates in aqueous solution and the low toxicity of their constituent monomeric organosilanes offers an advantage over other scaffolds in vivo, preventing bioaccumulation and saturation of complementary receptors (lectins). Despite the hydrolysis in water, the octamannosyl-POSS studied shows a 50-fold higher binding affinity to Con A than methyl α-d-mannopyranoside. These experiments suggest that the novel glyco-POSS are attractive compounds for in vivo applications that require multivalent display of glycans.
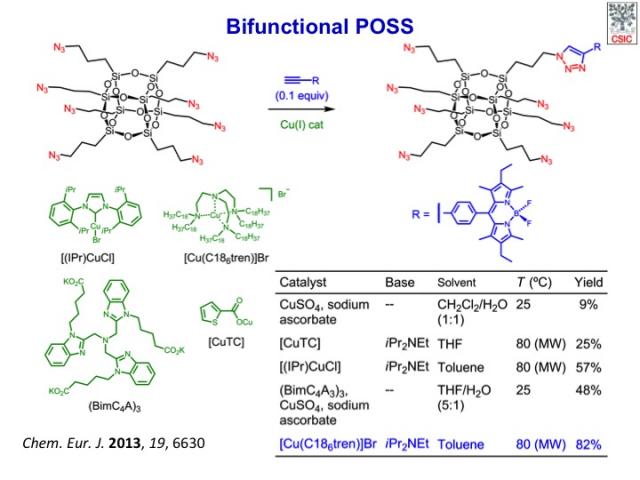
We have described a general procedure for the assembly of hetero-bifunctional cubic silsesquioxanes with diverse functionality and a perfectly controlled distribution of functional groups on the inorganic framework. The method is based on a two-step sequence of mono- and hepta-functionalization through the ligand-accelerated copper(I)-catalyzed azide–alkyne cycloaddition of a readily available octaazido cubic silsesquioxane. The stoichiometry of the reactants and the law of binomial distribution essentially determine the selectivity of the key monofunctionalization reaction when a copper catalyst with strong donor ligands is used. The methodology has been applied to the preparation of a set of bifunctional nano-building-blocks with orthogonal reactivity for the controlled assembly of precisely defined hybrid nanomaterials and a fluorescent multivalent probe for application in targeted cell-imaging. The inorganic cage provides an improved photostability to the covalently attached dye as well as a convenient framework for the 3D multivalent display of the pendant epitopes. Thus, fluorescent bioprobes based on well-defined cubic silsesquioxanes offer interesting advantages over more conventional fully organic analogues and ill-defined hybrid nanoparticles and promise to become powerful tools for the study of cell biology and for biomedical applications.
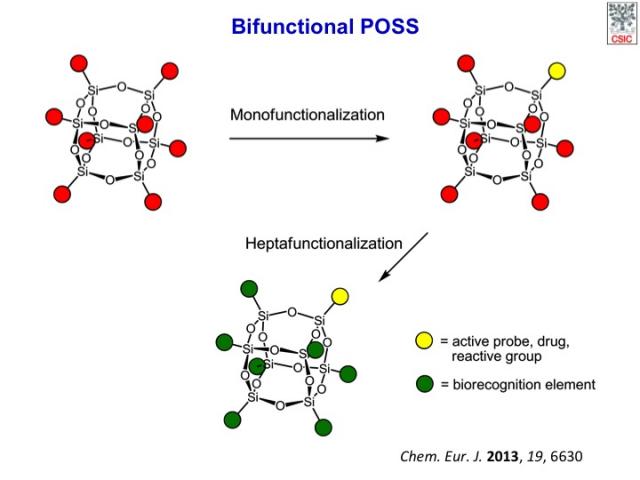
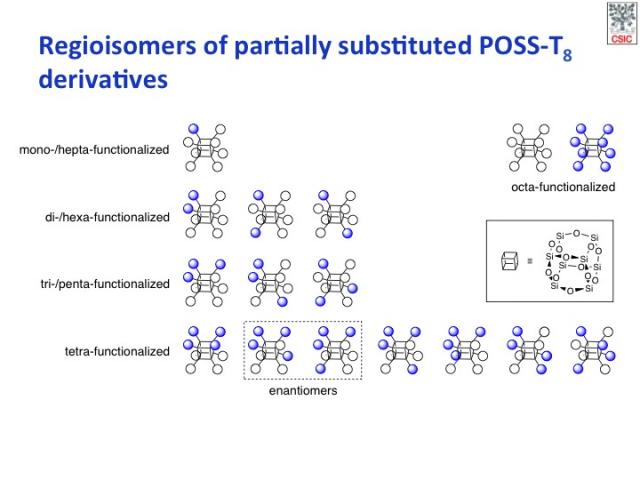
All possible POSS products and their different structural isomers (and stereoisomers) that can be generated in the reaction of a homo-octafunctional cubic POSS with a monofunctional reagent.
The selective synthesis of bifunctional POSS with well-defined substitution patterns is still a complex and largely unsolved challenge, in spite of recent progress.
Although the CuAAC monofunctionalization of octa-azido-POSS could in principle be achieved by simply performing the reaction with a large excess of POSS over alkyne, it is known that attempted CuAAC monofunctionalization of some polyazides can show unusual product distributions that deviate significantly from those expected on a purely statistical basis. This unusual result has been tentatively ascribed to direct participation of the initial Cu-triazolide intermediate in the subsequent click reaction of a vicinal-azide group.
The tetramine Br catalyst with added Hünig’s base in toluene as solvent and under microwave heating gave the highest yield of of monotriazolyl-POSS (82 %; Table), close to the expected statistical value (95.65 %).
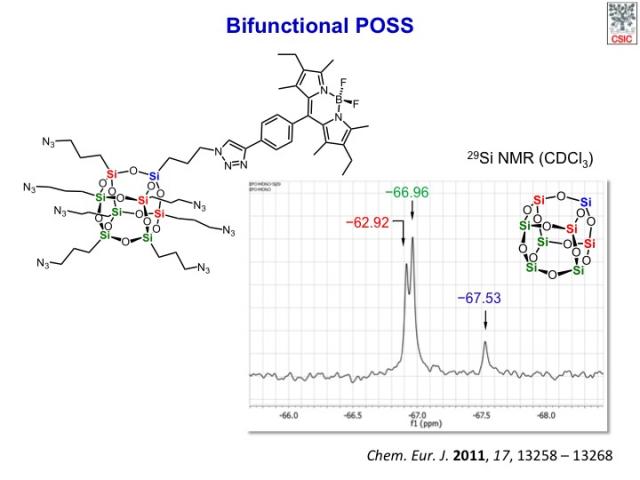
The degree of substitution of POSS products can be readily recognized by the 4:3:1 pattern distribution of 1H and 29Si NMR (Figure) signals, as expected from molecular symmetry considerations. The 29Si chemical shifts are within the expected region for an alkyl-substituted cubic POSS (ca. δ=−65 to −70 ppm).
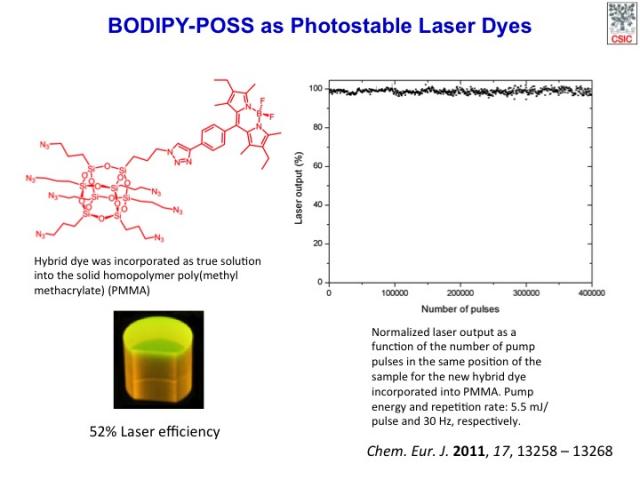
New hybrid organic–inorganic dyes based on an azide-functionalized cubic octasilsesquioxane (POSS) as the inorganic part and a 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BDP) chromophore as the organic component have been synthesized by copper(I)-catalyzed 1,3-dipolar cycloaddition of azides to alkynes. The monosubstituted new hybrid dye exhibits high lasing efficiency of up to 56 % with high photostability, with its laser output remaining at the initial value after 4×105 pump pulses in the same position of the sample at a repetition rate of 30 Hz. The new hybrid systems based on dye-linked POSS nanoparticles open up the possibility of using these new photonic materials as alternative sources for optoelectronic devices, competing with dendronized or grafted polymers.
Water-soluble fluorescently-labelled glyco-POSS was assayed as fluorescent probes for the imaging of C-type lectin receptors (CLRs) present on the surface of antigen-presenting cells by using fluorescence microscopy. An important group of CLRs recognize oligosaccharides containing mannose and/or fucose, including the mannose receptor (MR) and DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin, also called CD209) on dendritic cells (DC). DC-SIGN in particular has attracted much interest since its discovery in 2000, because it binds to a large range of clinically relevant pathogens, including HIV, Ebola virus, Candida albicans and Leishmania, among others, facilitating their uptake for subsequent antigen presentation.
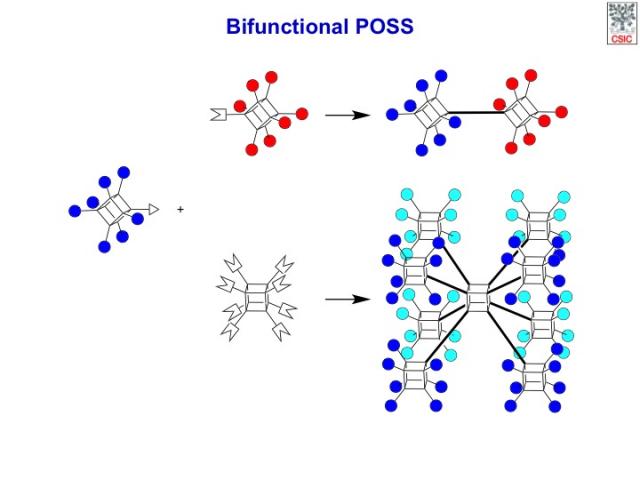
The obtained bifunctional POSS nano-building-blocks could be readily homo- or hetero-dimerized to afford, respectively, symmetric or asymmetric dumbbell-shaped POSS dyads, or they could be assembled with complementarily reactive homo-octafunctional-POSS monomers to produce even more complex 3D constructs.
Nonafluorobutanesulfonyl azide (nonaflyl azide): a stable, safe and cost-effective reagent for organic synthesis
We and others have recently shown that nonafluorobutanesulfonyl azide (nonaflyl azide: NfN3) is an efficient, shelf-stable and cost-effective diazo transfer reagent for the synthesis of azides from primary amines and for the preparation of α-diazo carbonyl compounds. More recently, we have developed a new procedure for the direct intermolecular C–H amination of simple hydrocarbons in the presence of a dirhodium(II) tetracarboxylate catalyst under mild thermal conditions.
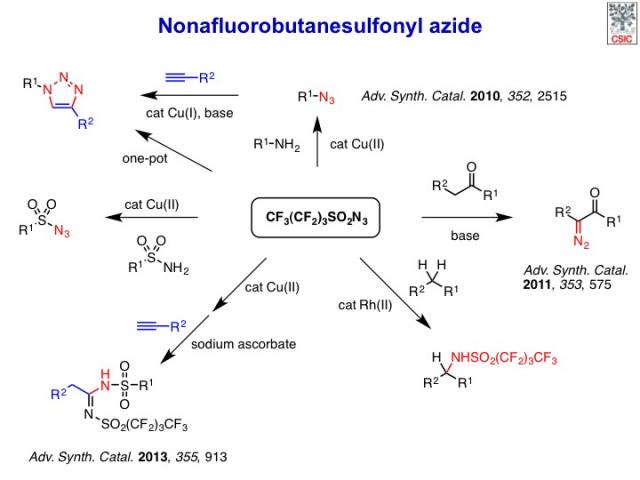
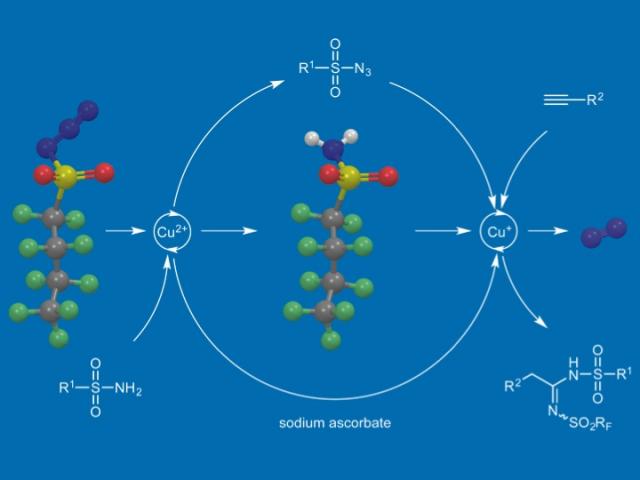
We have also explored the use of NfN3 for the synthesis of sulfonyl azides from readily available sulfonamides and the application of this reaction in a novel copper-catalyzed two-step, one-pot sequential process with added terminal alkynes to afford N,N′-disulfonylamidines.
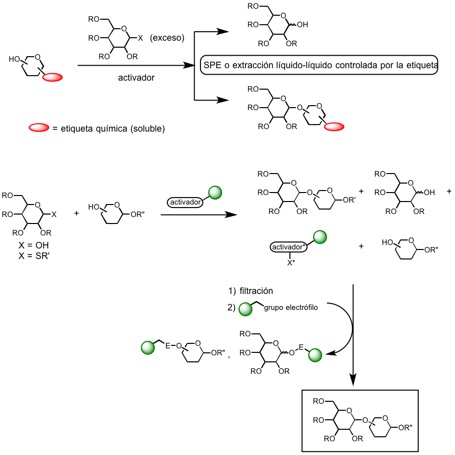
New methods and strategies for parallel solution-phase synthesis of oligosaccharides and glycoconjugates using solid supported and chemically tagged reagents
As a long term general objective of this research line, we intend to develop new practical and scalable synthetic methods that allow the preparation of complex oligosaccharides and glycoconjugates of biomedical interest, in a fast and simple way, which are amenable to be anchored to a polyfunctional support. In spite of recent accomplishments in solid-phase carbohydrate synthesis, this technique has a series of pitfalls that have preclude its widespread use: 1) the requirement of robust linkers, stable to a wide range of reaction conditions; 2) the difficulty in the analysis of the species attached to the solid support, and 3) the impossibility of purifying the intermediate compounds attached to the support in multistep synthesis. For this reason, in recent years new solution-phase techniques have been developed to ease reaction work-up and product isolation, avoiding the pitfalls of solid-phase approaches. In particular, we have as initial objective in this line the development of new reagents, scavenging agents, protecting groups and activating groups supported on insoluble polymers or chemically tagged with lipophilic or fluorophilic chains and their application to the preparation of natural oligosaccharides of biological interest using solution phase methods amenable to automation, greatly simplifying the purification steps.
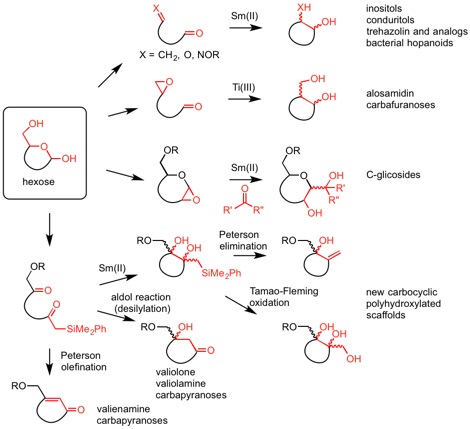
New methods of organic synthesis based on low valent lanthanide and transition metal reducing reagents
This line is aimed at the development of new carbon-carbon bond-forming processes via intra- or intermolecular reductive coupling reactions promoted by low valent titanium, vanadium, chromium or samarium reagents. These processes are in general highly stereoselective and, due to their radical character, take place under very mild conditions, being especially appropriate for the preparation of highly functionalized systems. In addition, these processes can be implemented into cascade reaction sequences, which combine radical and ionic steps. This methodology has been successfully applied to the synthesis of diverse natural products containing a cyclitol or C-glycoside substructure, which are active as glycosidase inhibitors, and to the preparation of new molecular scaffolds for the synthesis of glycomimetic libraries. Our methods have been successfully applied by several groups to the preparation of complex natural products.
Design and synthesis of glycomimetic compounds and their evaluation as specific enzyme inhibitors
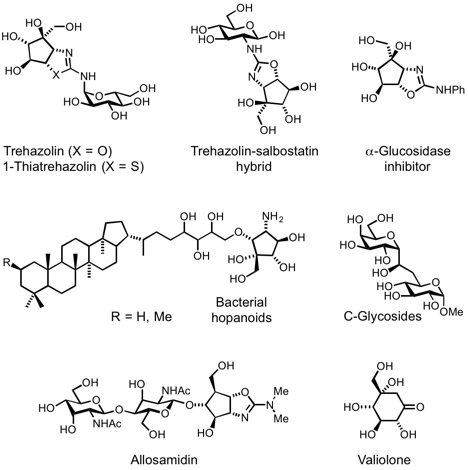
Carbohydrates and glycoconjugates are involved in a great variety of biological processes such as cellular development and differentiation, inflammation, fertilization, cancer metastasis, microbial infection, immune response and protein stability, folding and transport. The objective of this line of research is the design, synthesis and evaluation of substrate or transition state analogue inhibitors of enzymes involved in the biosynthesis and metabolism of carbohydrates and glycoconjugates, especially glycosidases. These inhibitors are highly useful as tools for the study of the reaction mechanism of the corresponding enzymes and the biological function of glycoconjugates, and as potential therapeutic agents for metabolic and infectious diseases. Our group has developed highly effective synthetic routes to several natural inhibitors of glycosylhydrolases as well as structurally modified analogs such as aminocyclitols and C-glycosides.