Transition metal-free cyclobutene rearrangement in fused naphthalen-1-ones: controlled access to functionalized quinones
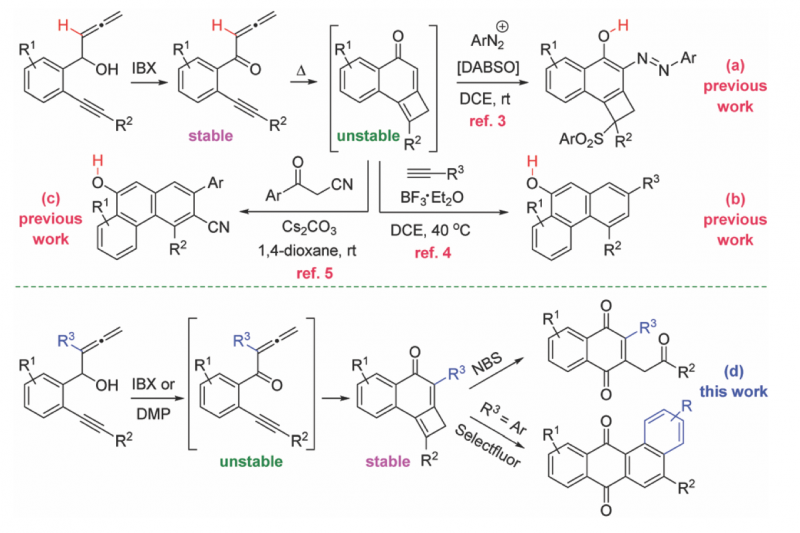
The ubiquity of the quinone moiety in natural products and organic materials justifies the continued interest in the synthesis of molecules containing this framework.1 The widespread occurrence of the cyclobutene nucleus in natural products and bioactive compounds, coupled to the use of this strained carbocycle as a building block in organic synthesis, triggered a renewed activity in the synthesis of cyclobutenes.2 However, the preparation of the quinone core from cyclobutenes has remained unexplored. Jiang and co-workers have recently communicated the cyclization reactions of allenynones toward naphthols using DABSO [DABCO·(SO2)2] and arenediazonium salts (Scheme 1a),3 or either in presence of alkynes (Scheme 1b),4 or β-ketonitriles (Scheme 1c),5 involving the generation of tricyclic cyclobutene intermediates. Aiming to extend the utility of allenynones, we planned to use allenyne precursors bearing substituents at the internal allene double bond. Worthy of note, the presence of the extra-substituent (R3 = Me, Ar) did allow for the isolation of previously unstable and non-isolable tricyclic cyclobutenes (R3 = H). Herein, we present a convenient method for the divergent synthesis of 1,4-naphthoquinones and tetraphene-7,12-diones through the oxidative reorganization of cyclobutene-fused naphthalen-1-ones.




