Research
Our group works in the areas of metal-free and transition metal catalyzed processes, electrochemistry and supramolecular organic chemistry. We are interested in developing new sustainable synthetic methodologies to access functional and complex structures in a controlled and selective fashion. These synthetic methods have been applied in the selective functionalization of drugs and bioactive molecules, as well as in the synthesis of natural products. A second pillar of our research focuses on the synthesis, characterization, self-assembly and bioactivity of amphiphilic molecules, within the area of systems chemistry. Our goal is to access functional self-assembly species resembling fundamental characteristics of living organisms.
Catalysis
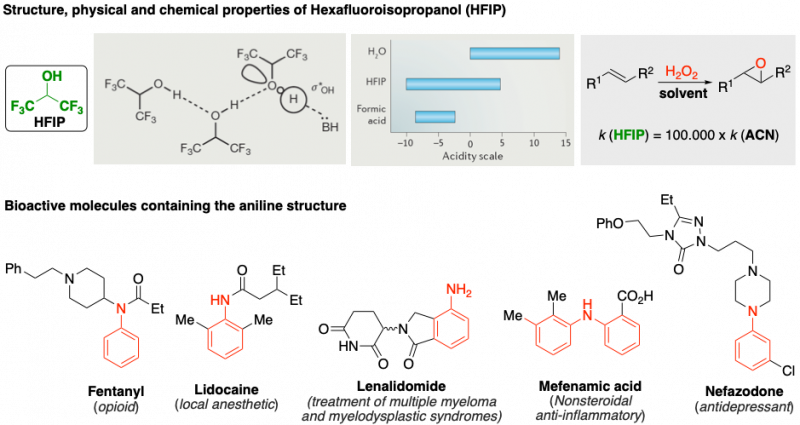
We have identified Hexafluoroisopropanol (HFIP) as a powerful solvent with unique properties that allows for interesting applications. For example, alkene epoxidation using hydrogen peroxide is 100.000 times faster in HFIP than in 1,4-dioxane because of the exacerbated acidity of HFIP as a result of the Hydrogen bond network stablished.
This prompted us to search for new modes of activation that HFIP could promote, focusing on amines, and particularly in anilines. Aniline containing bioactive molecules represent an important and heterogeneous toolbox to target a diverse array of medical issues. Developing novel and selective methods for the functionalization of anilines is highly desirable to expand such a collection of active molecules.
Nat. Rev. Chem., 2017, 1, 0088.
DOI: 10.1038/s41570-017-0088
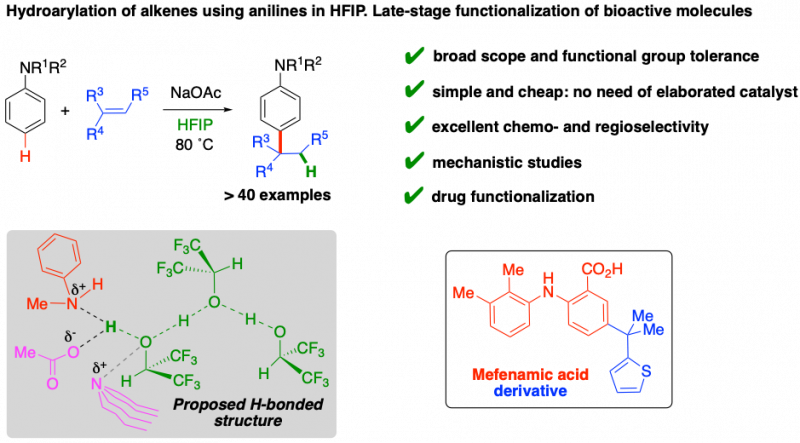
We have recently developed a new method for the selective functionalization of anilines using alkenes as electrophiles. It is an extremely atom-economic catalytic system, where HFIP both protonates the alkene and selectively enables anilines toward the electrophilic aromatic substitution. Mechanistic experiments allowed us to propose a hydrogen bonded structure as a key species in the process. This is a very simple and inexpensive method that has been applied to the selective derivatization of the nonsteroidal anti-inflammatory Mefenamic acid.
ACS Catal. 2020, 10, 6023–6029
DOI: 10.1021/acscatal.0c00872
Systems chemistry
Alternatively, we are involved in a challenging program aiming to build a synthetic protocell based on chemical reactivity principles. A protocell can be considered a self-replicating unit in which a genetic polymer and the components of its boundary undergo spatio-temporally coupled growth and replication under far-from-equilibrium conditions.
This ambitious project follows a bottom-up program to generate a toolbox of chemical reactions that can be used to build up increasingly complex dynamic systems, that could mimic important features of living organisms, such as compartmentalization, metabolism or far-from-equilibrium regime. We are particularly interested in using aminoacids and nucleobases as the basic building blocks. Although great advances have been made in peptide engineering, identifying small (lipo)peptides with defined supramolecular behaviour, catalytic properties or biological activity is still a challenge.
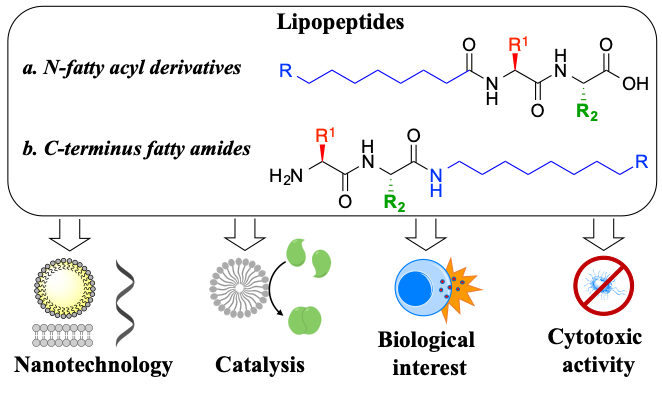
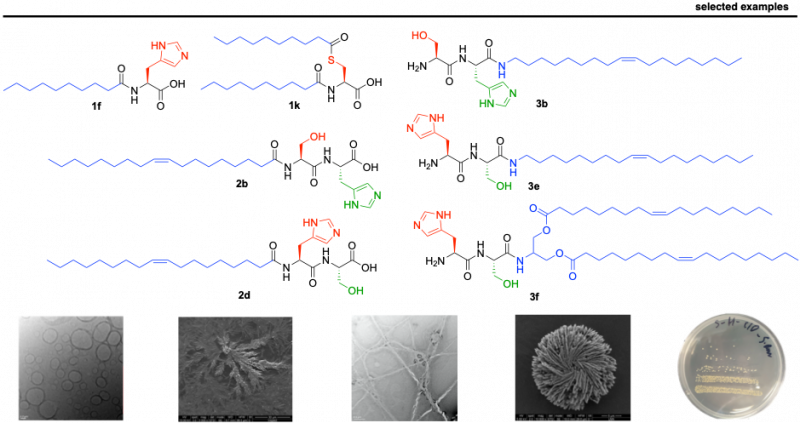
We have recently reported a library of short lipopeptides, together with their supramolecular characterization and antimicrobial activity against both Gram-negative (E. coli) and Gram-positive (S. aureus) strains. While simple lipoamino acids self-assemble into micellar or vesicular structures, incorporating dipeptides capable of stablishing hydrogen bonds results in advanced fibrilar structures. We have studied their supramolecular behaviour, Critical Aggregation Concentration (CAC), particle size (using Dynamic Light Scattering - DLS), stability (zeta-potential) and morphology (using Transmission and Scanning Electron Microscopy – SEM and TEM). The self-assembly effect has proven to be key to achieve antimicrobial activity
Org. Biomol. Chem. 2021, 19, 6797–6803
DOI: 10.1039/D1OB01227D




